FDA panel unanimously recommends Moderna Covid booster shots for at-risk adults
لجنة الدواء والغذاء الأمريكية توصي بجرعة ثالثة من موديرنا لكبار السن المعرضين للخطر
CNBC
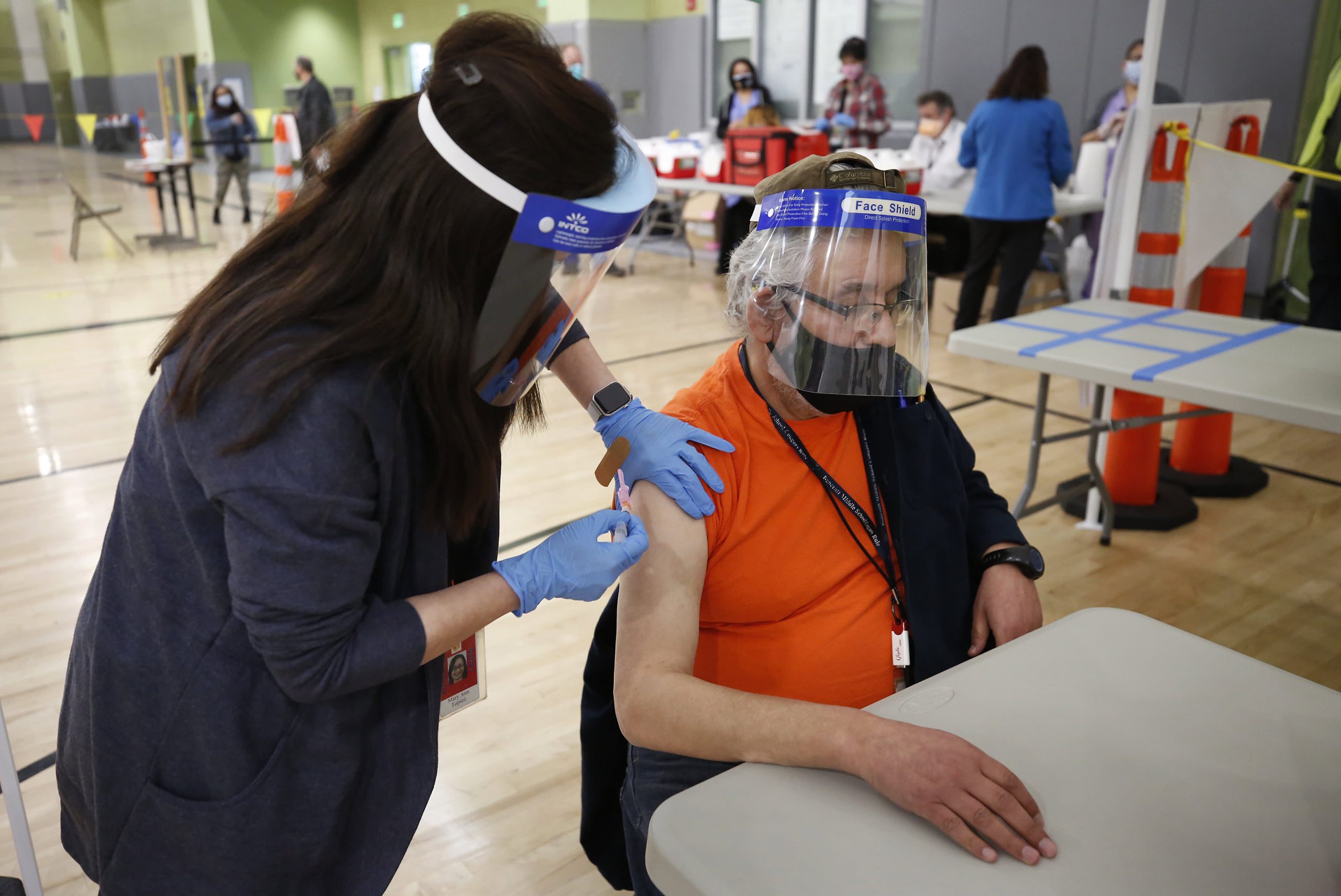
A key Food and Drug Administration advisory committee on Thursday unanimously recommended giving booster shots of Moderna’s Covid-19 vaccine to people ages 65 and older and other vulnerable Americans. The vote was a crucial step before the U.S. can start administering third shots to some of the more than 69 million people who originally received that vaccine.
The nonbinding decision by the FDA’s Vaccines and Related Biological Products Advisory Committee would bring guidelines for Moderna in line with third shots of the Pfizer-BioNTech vaccine. Those shots were authorized less than a month ago to a wide array of Americans, including the elderly, adults with underlying medical conditions and those who work or live in high-risk settings, such as health-care and grocery workers.
While the agency hasn’t always followed the advice of its committee, it often does. A final FDA decision on Moderna boosters could come within days. A CDC vaccine advisory committee is then expected to vote on the FDA’s proposal next week. If it recommends approval and the CDC endorses it, booster shots could begin immediately for eligible Americans who completed their immunizations at least six months ago.
Booster shots have been a contentious topic for scientists — in and outside the government — especially as many people in the U.S. and other parts of the world have yet to receive even one dose of a vaccine. The World Health Organization is urging wealthy countries to hold off on distributing boosters, and some scientists say they aren’t convinced most Americans need boosters right now.
When the FDA committee met last month, they rejected a proposal to distribute booster shots of Pfizer and BioNTech’s vaccine to the general public. Some committee members at the time said they were concerned there wasn’t enough data to make a recommendation, while others argued third shots should be limited to certain groups.
After Moderna’s unanimous vote Thursday, committee member Dr. Patrick Moore said the data the company submitted for authorization of a booster “was not well explained,” adding he voted yes more on “gut feeling.”
“The data itself is not strong, but it is certainly going in a direction that is supportive of this vote,” he said.
Some members said the boosters should prevent so-called breakthrough infections, which they said is critical for protecting health-care institutions from becoming overwhelmed, while other members said the third shots should ensure those at high risk won’t suffer from severe disease. Some committee members also suggested young people may not need boosters, as the initial shots are still holding up in those groups.
Dr. Paul Offit, another member, stressed that most people who have received the first two doses of Moderna’s vaccine are still well protected and said he hopes the recommendation doesn’t send the “wrong message” to the general public.
“If we’re trying to prevent what is inevitable, which is a decline in neutralizing antibodies and an erosion in protection against mild or asymptomatic infection, that is a high bar to which we hold no other vaccine,” he told his colleagues.
The Biden administration hopes giving the U.S. population additional doses will ensure long-term and durable protection against severe disease, hospitalization and death as the fast-moving delta variant continues to spread.
Dr. Peter Marks, the FDA’s top vaccine regulator, addressed the committee Thursday before the vote, telling the panel of experts that the agency encourages “all the different viewpoints” regarding the “complex and evolving” data.
“That said, as we proceed, I would ask that we do our best to focus our deliberations on the science related to the application under consideration today, and not on operational issues related to a booster campaign or issues related to global vaccine equity,” he added.
Moderna applied for FDA authorization of a booster dose on Sept. 1. The company said the results are based on a clinical trial of roughly 170 adults, fewer than the 318 people studied for Pfizer’s booster. Moderna said a third shot at half the dosage — 50 micrograms — used for the first two jabs was safe and produced a strong immune response.
Upon approval, the company plans to send a letter to health-care providers explaining the difference in dosage for the third shot, Dr. Jacqueline Miller, the company’s head of infectious disease research, said during a presentation Thursday.
Side effects of Moderna’s boosters were comparable with those experienced after the second dose, the company wrote in a document released Tuesday by the FDA. Most adverse reactions were low in severity, and Moderna reported no cases of a rare heart inflammation condition, myocarditis or pericarditis, in trial participants up to 29 days after they received their boosters.
Before recommending the third shots, the panel listened to multiple presentations, including from health authorities from Israel, which began offering boosters to its population ahead of many other countries. The country has used mostly Pfizer’s vaccine, but some Moderna boosters have been given.
Israel has administered 3.7 million third shots since it began its booster campaign in late July, with roughly a third of the extra shots going to people age 60 and older, Sharon Alroy-Preis, director of public health services at Israel’s Ministry of Health, told the panel.
She presented data that suggested people who receive a booster dose were less likely to get infected with Covid or become severely sick. She said officials have so far identified 17 cases of myocarditis or pericarditis following third doses.
“I think we can say when we’re looking at all the data in Israel so far is that the administration of booster doses helped Israel lessen the infections and the severe cases,” she said.
الشرق
أوصت استشارية رئيسية تابعة لإدارة الغذاء والدواء الأميركية، أمس، بالإجماع إعطاء جرعات معززة من لقاح Covid-19 من موديرنا.
وأشارت هيئة الغذاء والدواء أن الفئات المسموح لها أخذ جرعة اللقاح المعززة هي كبار السن الذين تبلغ أعمارهم أكثر من 65 عاماً والفئات الأخرى المعرضة للخطر، وفقاً لـ سي ان بي سي.
وسبق هذا القرار بشأن موديرنا قرار مماثل يخص لقاح فايزر المضاد لفيروس كورونا.
وتلقى الملايين، ممن حصلوا مسبقا على جرعتين من لقاح فايزر، منذ ستة أشهر على الأقل، على جرعة ثالثة معززة من فايزر.
وستناقش لجنة إدارة الغذاء والدواء الأميركية إعطاء جرعات معززة من لقاح جونسون آند جونسون يوم الجمعة.
ومن المتوقع صدور قرارات نهائية الأسبوع المقبل.
ويشدد المسؤولون الأميركيون على أن الأولوية هي إعطاء اللقاح إلى 66 مليون أميركي غير محصنين إلى الآن.